Selling Globally Starts With Compliance
From our certified production facility in the EU, we manufacture products that are designed to meet the strictest international quality and safety standards:
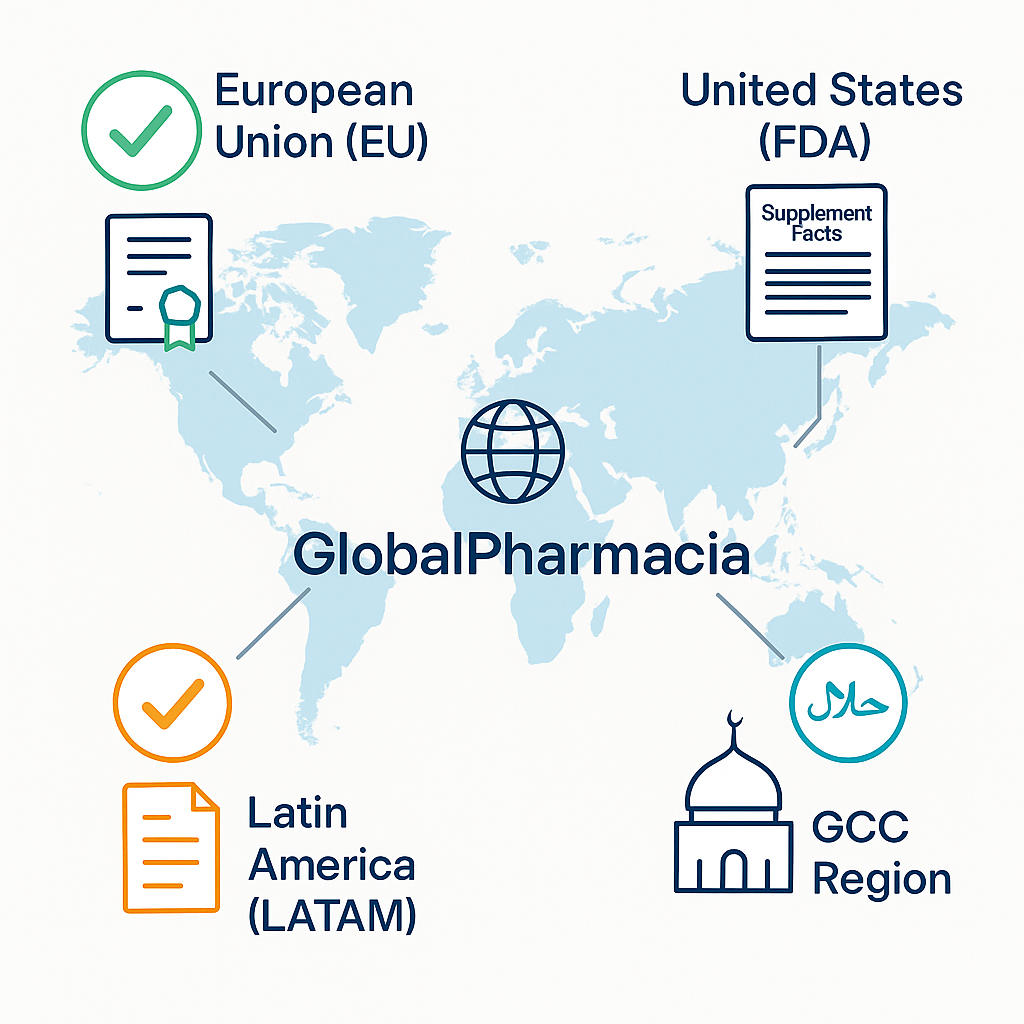
✅ European Union (EU)
- Full compliance with Regulation (EU) 2015/2283 for novel foods
- Organic production per Regulation (EU) 2018/848
- Labeling, ingredient limits, and EFSA recommendations
✅ United States (FDA)
- Support for cGMP (21 CFR Part 111) standards
- Supplement Facts panel formatting and ingredient documentation
- Claims guidance based on DSHEA and FTC guidelines
✅ GCC Region (Saudi Arabia, UAE, etc.)
- Halal-certified production options
- Registration documents and conformity for GSO/GCC SFDA regulations
- Arabic labeling support when required
✅ Latin America (LATAM)
- Ingredient compliance and dosage limits per ANVISA (Brazil), COFEPRIS (Mexico), INVIMA (Colombia), and others
- Regulatory dossiers and export-ready certification packages
At GlobalPharmacia, we don’t just manufacture—we make sure your product meets the regulatory standards of your target market. With compliance support spanning the EU, US, GCC, and LATAM regions, our team ensures your documentation, labeling, and formulas are ready for export success.
What We Provide
At GlobalPharmacia, our team helps you prepare everything you need for successful market entry:
- Product specifications and certificates (GMP, HACCP, ISO 22000, Organic)
- Ingredient safety data and technical sheets
- Label templates adapted to your market
- Export documentation and batch traceability
We act as your regulatory partner, so you don’t need to navigate these complexities alone.
Ready to Go Global?
With partners in 30+ countries and experience across multiple regulatory landscapes, we’re equipped to help your product pass inspections, registrations, and customs—smoothly and efficiently.
📩 Contact our team to learn how we can prepare your product for success—anywhere in the world.